Home / Publications / Research / Access and Affordability: Building Fiscally Responsible Pharmacare Systems
- Media Releases
- Research
- |
Access and Affordability: Building Fiscally Responsible Pharmacare Systems
Summary:
| Citation | Rosalie Wyonch. 2025. "Access and Affordability: Building Fiscally Responsible Pharmacare Systems." ###. Toronto: C.D. Howe Institute. |
| Page Title: | Access and Affordability: Building Fiscally Responsible Pharmacare Systems – C.D. Howe Institute |
| Article Title: | Access and Affordability: Building Fiscally Responsible Pharmacare Systems |
| URL: | https://cdhowe.org/publication/access-and-affordability-building-fiscally-responsible-pharmacare-systems/ |
| Published Date: | September 18, 2025 |
| Accessed Date: | November 27, 2025 |
Outline
Outline
Related Topics
Press Release
Files
For all media inquiries, including requests for reports or interviews:
Introduction and Overview:
-
This Conference Report captures findings from a C.D. Howe Institute workshop of policymakers, industry leaders, and health experts. Participants agreed that federal pharmacare funding should focus on reducing coverage gaps and out-of-pocket costs for the uninsured and underinsured, rather than replacing Canada’s mixed public-private system. This approach avoids disruption for the 27 million Canadians with private coverage and eases fiscal pressures.
-
With almost all of health budgets locked into legacy costs, provinces have little room for new large-scale programs. Targeted reforms – such as enhancing catastrophic coverage, expanding existing provincial programs to fill access gaps, or Quebec-style mixed models – were seen as more financially sustainable than universal first-dollar coverage.
-
Rushed implementation, administrative complexities, and limited government expertise in drug distribution supply chains and benefit management pose real risks for unintended disruptions. Stronger coordination with private insurers, clearer public communication, and better provincial integration were highlighted as keys to success.
-
Along with the Canada Drug Agency reports on a national procurement strategy and formulary due this fall, the federal government should clarify its intentions for the future of pharmacare policy. The current framework is underfunded, and new bilateral agreements are paused, creating significant uncertainty. Current legislation should be reformed to support mixed universal coverage models to improve the fiscal sustainability and flexibility for provincial autonomy in expanding coverage.
Agenda:

Agenda:

Rapporteur’s Summary:
By Rosalie Wyonch
The C.D. Howe Institute thanks those who provided comments or signed off in support of an earlier draft: Neil Fraser, Fred Horne, Joe Farago, IMC, Joan Weir, CLHIA, Jeff Beach, Asthma Canada, David Veillette, McKesson Canada, Gary Walters, Smart Health Benefits Association, Simona Zar, CAPDM.
Introduction and Context
In August 2025, healthcare policy stakeholders convened in Toronto to address one of Canada’s most pressing healthcare policy challenges: developing a fiscally responsible approach to universal pharmacare that balances accessibility, affordability, and sustainability.11 Canada’s universal healthcare system does not include prescription drug coverage, but all provinces have public drug insurance programs. Each province covers different medications, and many have varying coverage levels depending on a number of sociodemographic factors such as age, household income, and employment status. The workshop, hosted by the C.D. Howe Institute, brought together government officials, industry representatives, academics, and policy experts to examine the current state of pharmaceutical coverage in Canada and chart a path forward for evidence-based reform.
The timing of this gathering was particularly significant, occurring amid a shifting federal political landscape and growing uncertainty about the future direction of Canada’s pharmacare policy. With bilateral agreements already in place in several provinces and mounting fiscal pressures at all levels of government, participants sought to move beyond ideological debates toward pragmatic solutions that could address genuine coverage gaps without disrupting existing systems that serve Canadians well.
The workshop’s foundation rested on presentations of compelling empirical evidence, different industry perspectives and the views of experienced experts in public administration and governance. Overall, the primary challenge lies in addressing specific gaps affecting vulnerable populations, particularly those facing high out-of-pocket costs, with low incomes, seniors, and individuals in precarious employment situations, rather than addressing a broad population need.
The proceedings revealed remarkable consensus among diverse stakeholders around several core principles. First, participants unanimously supported a “fill the gaps” approach that would target resources toward Canadians without coverage and address underinsurance issues, rather than implementing a comprehensive single-payer system. In a single-payer system, the government would be the sole insurer of drugs included in the public plan formulary for the whole population (there would still be a role for private insurance companies to cover medications approved for the Canadian market, but not publicly insured). A “fill the gaps” approach minimizes disruption for the approximately 27 million Canadians with existing private coverage and fiscal liability for the public sector. This stance reflected both fiscal realities and recognition that private plans typically offer significantly broader formularies and faster access to innovative treatments than public programs.
Many presenters and participants emphasized the importance of maintaining provincial jurisdiction while enabling federal financial support through bilateral arrangements. Quebec’s pharmacare system emerged as a frequently cited model, demonstrating how universal coverage could be achieved while preserving private insurance options and maintaining fiscal sustainability through structured cost-sharing mechanisms.
Industry stakeholders highlighted critical implementation challenges that should be addressed in future expansions or changes to public coverage. Insurance representatives detailed how fragmented claims data disrupts automated processes for plan members and employers, while pharmaceutical industry participants raised concerns about commercial predictability and Canada’s attractiveness as a launch market for innovative therapies. Distribution sector representatives noted often-ignored supply chain implications, particularly how reduced drug pricing could threaten physical accessibility in remote communities where distribution margins are already thin.
The sessions revealed significant knowledge gaps within government regarding drug distribution systems, pricing mechanisms, and the practical complexities of benefit administration. These knowledge gaps create significant implementation risks for Canadians accessing medications through these programs. Former government officials acknowledged that bureaucratic constraints often limit strategic thinking about drug programs, which are typically managed as budget line items rather than portfolios of technology requiring long-term strategic management.
Participants converged on several actionable recommendations: enhanced communication about existing coverage options, targeted financial support for identified gaps across public plans, preservation of successful provincial programs while enabling federal supplementation for emerging therapies and rare diseases. The workshop concluded with broad agreement that incremental, evidence-based improvements to existing systems would better serve Canadians than wholesale transformation toward a single-payer model, particularly given current fiscal constraints and the proven effectiveness of Canada’s mixed public-private approach to pharmaceutical coverage.
Universal Pharmacare Coverage in Canada – Defining the Problem
The opening session established a comprehensive empirical foundation through the presentation of two complementary research analyses examining Canada’s pharmaceutical coverage landscape. The first presentation introduced findings from the Gaps 2.0 study, a quantitative analysis providing measurement of prescription drug insurance coverage across Canadian provinces.22 This research methodology involved systematic examination of publicly available plan information documents published by the Canadian Institute for Health Information, establishing eligibility criteria across all jurisdictions and matching these parameters with population demographics while incorporating private coverage data representing approximately two-thirds of the Canadian population. The second presentation provided an economic and fiscal perspective, including projections of various pharmacare scenarios through 2030, and highlighting issues important to the current policy context.
The analysis revealed that 97.2 percent of Canadians possess access to some form of prescription drug coverage, fundamentally challenging prevailing assumptions about coverage gaps (Figure 1). The research demonstrated that 65 percent of Canadians maintain private coverage, while 66 percent qualify for public program eligibility, with 40 percent actively enrolled in public plans. This leaves only 2.8 percent of the population without any coverage access, concentrated primarily in specific demographic pockets within Ontario and Newfoundland. The presentation emphasized that these proportions, based on 2020 population data, remained applicable since overall plan designs had not undergone significant structural modifications.
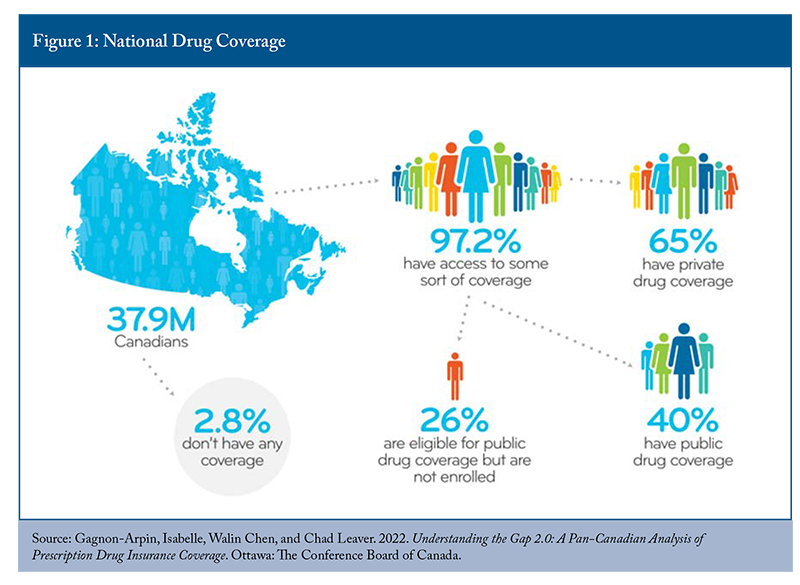
Significant attention was devoted to reconciling these findings with widely circulated Statistics Canada survey data suggesting that one in five Canadians lacks prescription drug coverage. The presentation attributed this discrepancy to methodological limitations in self-reported surveys, particularly the specific question asking whether respondents possessed insurance covering “at least some” prescription medication costs within the preceding twelve months. Multiple factors could generate negative responses despite actual coverage availability, including no prescription needs during the survey period, formulary limitations excluding specific required medications, or a limited understanding of available public program options.33 According to Statistics Canada’s analysis of the Canadian Community Health Survey, about 89 percent of people in Quebec and slightly more than three-quarters of residents of British Columbia had some form of prescription drug coverage (Yang and Gupta 2024). During the discussion, participants raised the issue of public perception and the apparent paradox between documented universal coverage availability in provinces like British Columbia and Quebec and substantial survey respondent populations reporting inadequate or no coverage. Participants explored whether this represented fundamental policy design limitations or implementation and awareness gaps that could be addressed through enhanced public education and program accessibility improvements.
The presentations turned to examining the eligible but non-enrolled populations, representing 26 percent of Canadians qualified for public coverage who choose not to participate. Contributing factors included existing private coverage satisfaction, insufficient awareness of public program availability, cost-benefit calculations where premiums exceeded anticipated medication expenses, and the absence of current pharmaceutical needs among healthy younger demographics. This finding suggested substantial policy intervention potential through improved program design and enhanced public communication rather than comprehensive system restructuring.
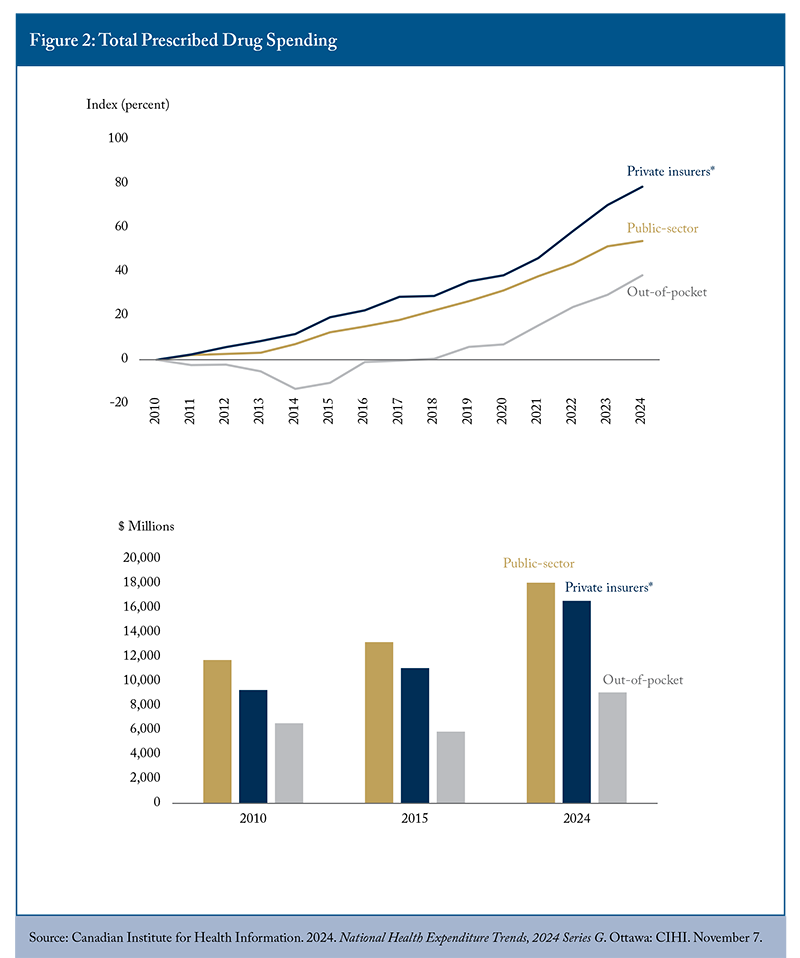
Prescribed drug spending trends across public, private, and out-of-pocket segments show increasing spending across all categories (Figure 2). Increasing prescribed drug spending is driven by many factors, including population growth and higher utilization related to an ageing population. Both public and private insurance plans show a growing proportion of spending being dedicated to high-cost treatments (over $10,000 per patient) for relatively few beneficiaries. Private insurance spending has covered a larger share of prescribed drug spending over time, while the share of public sector and out-of-pocket spending has declined. These trends indicated progress in reducing individual financial exposure while transferring responsibility to primarily employment-based coverage arrangements.
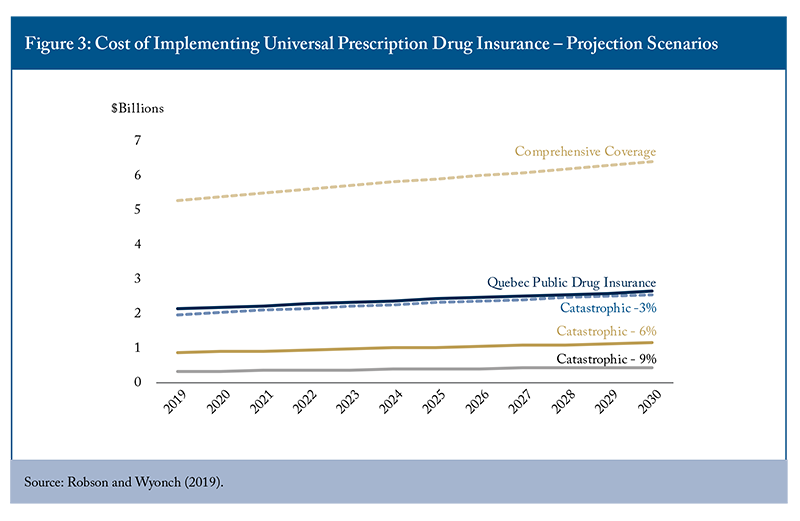
Catastrophic coverage analysis examined household spending burdens across different income quintiles and demographic segments, revealing that lower-income households and seniors faced disproportionate pharmaceutical cost challenges. All provinces have prescription drug coverage programs for at least some segments of the population, including low-income households and seniors. There are also programs to limit the total cost of medications, though there is significant interprovincial variation in threshold levels and eligibility criteria (Table 1). Economic modelling compared various universal coverage approaches, including catastrophic backstops at different income percentage thresholds, comprehensive provincial formulary expansion, and mixed public-private arrangements incorporating premiums and copayments (Figure 4).

Cost projections indicated significant fiscal implications for different policy approaches, with fill-the-gaps comprehensive coverage expansion representing the most expensive option (though still less than half as expensive as first-payor universal comprehensive coverage).44 The Parliamentary Budget Office estimated that single-payer universal coverage based on the Quebec formulary would result in incremental costs of about $11.2 billion in 2024-25, increasing to $13.4 billion in 2027-28 (Barkova and Busby 2023). In contrast, the Quebec model (which includes premiums and copayments) and catastrophic coverage models offered more financially sustainable alternatives. The analysis highlighted Quebec’s mixed model as the more fiscally prudent option for achieving universal pharmacare coverage.55 Notably, Quebec regulates minimum coverage levels for private insurance in addition to public coverage, effectively creating a common universal minimum coverage level. Other provinces have shown little interest in regulating private insurance coverage in this manner. The Quebec model provides more comprehensive coverage than a catastrophic backstop on spending and is more fiscally sustainable than expanding similar coverage without the implicit cost recovery mechanisms.
The current policy and political landscape add complexity to the quantitative backdrop of addressing gaps in pharmacare access and insurance. There is significant uncertainty about the future of the existing approach to national pharmacare. While three provinces and one territory have signed bilateral agreements, there is no commitment from the federal government to finalize additional agreements with interested provinces and territories (Froese 2025). Meanwhile, Donald Trump’s May 12 executive order that US drug prices must not exceed the lowest price in other nations does not mention Canada explicitly, but could cause significant damage to our public healthcare system and ripple around the globe.66 The White House. 2025. “Delivering Most-Favored-Nation Prescription Drug Pricing to American Patients.” Executive Order. May 12. Washington, DC: The White House. https://www.whitehouse.gov/presidential-actions/2025/05/delivering-most-favored-nation-prescription-drug-pricing-to-american-patients/. The move could massively increase prices in Canada (and other reference nations) and create a significant incentive for pharmaceutical companies to delay launching new medicines in lower-priced markets (Wyonch 2025). The Canada Drug Agency is also in the process of developing the legislated reports on a national bulk purchasing strategy and formulary.
The discussion period generated substantial engagement around the quantitative results and policy implications of the presented research, as well as important nuances and factors to consider. Participants sought clarification regarding precarious employment impacts on coverage access, particularly concerning gig economy workers and youth unemployment effects on pharmaceutical access.
There was debate about the best role for the federal government to play in complementing provincial insurance coverage or encouraging its expansion. There was general agreement that bilateral agreements and maintaining provincial jurisdiction are a practical approach to overcoming the lack of consensus among provinces. The conversation addressed whether establishing national standards or templates could provide guidance for provincial policy development while respecting constitutional jurisdictional divisions. The discussion explored the existing medical expense tax credit framework, which provides deductions for healthcare spending exceeding three percent of household income, as a potential foundation for establishing a national threshold.
Questions emerged regarding the temporal urgency of policy intervention, with participants debating whether current provincial expansions and bilateral agreements could provide practical insights for other provinces to improve efficiency and smooth implementation in the future or whether more systematic coordination was required. The discussion acknowledged ongoing live experiments in several provinces while expressing concern about the disruption of patient coverage, resulting in confusion, and added burden on pharmacists and physicians in providing prior authorization and explaining changes to patients. In addition, provinces are likely weary of time-limited federal funding commitments and uncertainty about future program funding and sustainability.
Participants raised concerns about opportunity costs associated with different policy approaches, questioning whether public resources might achieve greater health system improvements through alternative investments in primary care access, surgical capacity, or diagnostic services rather than pharmaceutical coverage expansion. This discussion emphasized the importance of a comprehensive health system perspective rather than an isolated pharmacare focus when evaluating policy priorities and resource allocation decisions.
The session concluded with recognition that effective pharmacare policy development required a nuanced understanding of existing coverage complexities, careful attention to provincial variation and autonomy concerns, and a strategic focus on addressing genuine access gaps rather than pursuing ideologically driven comprehensive system transformation that might disrupt successful existing arrangements serving the majority of Canadian patients effectively.
Public and Political Perception: Implications for Pharmacare and Fiscal Sustainability
The presentations provided perspectives from the insurance industry and former public administrators on the financial and political nuances surrounding expansions to public pharmacare policy. The first presentation highlighted survey results about private insurance coverage and public perception. Approximately 27 million Canadians currently possess private drug coverage, with 88 percent of those with a benefits plan placing value on having access to their existing coverage, and 84 percent recognize significant cost savings through their plans (Coletto 2023).77 Survey results show that the average household saved about $913 in prescription drug costs as a result of having a private insurance plan. Survey results show that most people (65 percent) preferred targeting help to those without insurance over covering the entire population.
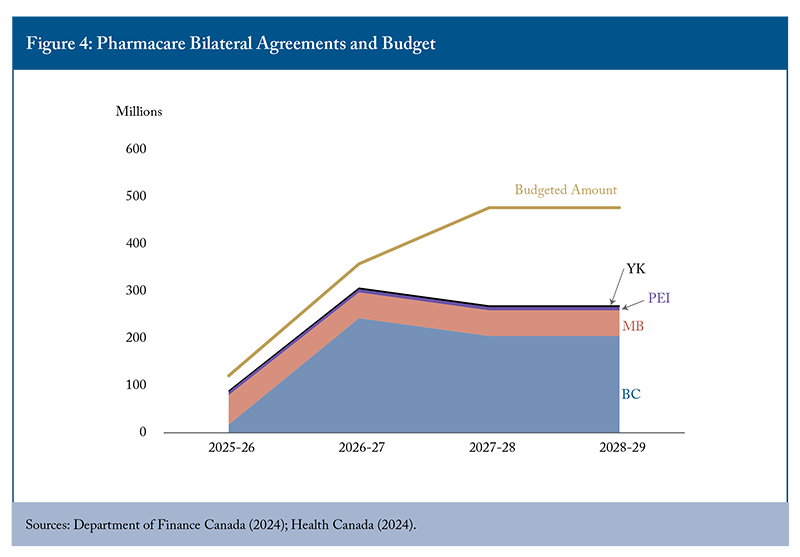
The presentations also highlighted uncertainty about program costs and implementation complexities. The 2024 federal budget allocated $1.5 billion over five years for diabetes and contraception coverage. The Parliamentary Budget Office (PBO), however, estimates that the five-year cost to the federal government will be $1.9 billion (Barkova 2024). This estimate does not factor in behavioural and market responses such as switching from uncovered to covered medications and assumes that existing provincial and private coverage will remain unchanged. In reality, the cost burden of covered medications has completely shifted to the public in Manitoba and Prince Edward Island (the provinces that have launched their new pharmacare programs), since insurers are not allowed to provide duplicative insurance. The three provinces and one territory with bilateral pharmacare agreements represent only 18 percent of the Canadian population, but cover 65 percent of allocated federal funding (Figure 4). The estimated costs of initial pharmacare implementation were overly optimistic, and the federal government under-budgeted for pharmacare rollout below that estimate. Only 35 percent of the budget remains for bilateral agreements with all remaining provinces. It is simply unrealistic for universal first-dollar coverage to be achieved with the current federal budget spending plans.
Implementation challenges in provincial rollouts illustrated systemic challenges that could be addressed through cooperation with private insurance providers, improved process automation, and public awareness campaigns. For example, as patients switched from private insurance plans to the universal public program, prior authorization required for some treatments to be reimbursed did not automatically transfer. As a result, some patients had disruptions to their coverage, and they needed to submit new authorization. This could be avoided by having private insurance companies administer public insurance. Rapid rollouts of the new program can also create an administrative burden for pharmacy and insurance providers who need adequate time to adapt their claims systems and formularies to complement public first-dollar coverage (the presentation noted that a complete Drug Identification List for the public plan formulary in Manitoba was publicly available only days before program launch).88 Doctors Manitoba. 2025. “Pharmacare Changes.” April 17. https://doctorsmanitoba.ca/news/pharmacare-changes. During the discussion, some participants noted that insufficient consultation during policy development phases can result from rushed implementation timelines related to political cycles rather than evidence-based planning.
The presentation emphasized superior private sector performance in program administration, citing faster listing procedures for novel medications, streamlined prior authorization processes, and enhanced customer service capabilities compared to government programs.
The next presenter provided a political policy perspective and highlighted the fiscal constraints on new health program spending at the provincial level. Between 98 and 99 percent of health budgets consist of predetermined legacy commitments and existing program delivery costs, leaving minimal discretionary spending for strategic initiatives. The presentation emphasized that drug programs function as budget-driven entities rather than strategically managed technology portfolios, constraining policy innovation and long-term planning capabilities. The urgency of a challenge tends to drive strategic health spending initiatives, and expansions to drug insurance must be considered against the opportunity costs to other essential services, including primary care access, surgical capacity, and diagnostic services. In the broader government context, healthcare represents the largest category of public spending and must be considered in balance with other public programs, such as education and justice. Little budget discretion and little room to grow the budget significantly constrain health ministers and policymakers in further expansions to public health programs.
Within pharmacare, policymakers should consider the trade-offs between investment in emerging therapies and treatments for rare diseases versus subsidizing (mostly) generic medications already covered through existing provincial programs or private insurance coverage. The presentation questioned whether current rare disease strategies had achieved meaningful implementation, noting prolonged delays between funding announcements and actual patient access to treatments.99 In 2023, the federal government allocated $1.4 billion in funding to the National Strategy for Drugs and Rare Diseases. All provinces except for Quebec have signed bilateral agreements with the federal government based on a set funding formula (Health Canada 2023).
Participants engaged extensively with sustainability and predictability concerns, emphasizing the importance of implementation strategies that avoid overnight disruption of existing coverage arrangements. The discussion highlighted the paradox of pursuing national standardization through provincial implementation, noting that existing bilateral agreements had produced divergent interpretation and application across jurisdictions, potentially exacerbating rather than resolving interprovincial variations. Each province, however, already has different formularies and pharmacare programs, meaning different gaps in access or pharmacare support already exist. Harmonizing across the country would require different strategies and changes in each province.
Industry representatives raised important concerns regarding commercial confidence and Canada’s attractiveness as a pharmaceutical market destination. Market uncertainty is already high due to economic and trade disruptions. The uncertain future of public pharmacare policy, along with its implications for private insurance, is undermining market incentives to launch new medications in Canada – ultimately disadvantaging patients who need access to cutting-edge therapies. This uncertainty is amplified by the emerging US drug policies.
Provincial autonomy emerged as a persistent and important theme, with participants questioning federal capacity to impose standardized approaches given constitutional jurisdictional divisions and political resistance to centralized health administration. The conversation explored potential mechanisms for achieving coordination without surrendering provincial decision-making authority, including enhanced federal transfers targeted toward specific therapeutic categories or patient populations.
Distribution and supply chain considerations received attention, with participants noting that pharmacy benefit management extends far beyond formulary decisions to encompass complex logistics, clinical support services, and patient education programs. The discussion emphasized that successful pharmacare implementation requires a comprehensive understanding of pharmaceutical ecosystems rather than a narrow focus on coverage decisions and financial transfers. A particularly important practical consideration relates to access and prices: if public insurance programs are successful in achieving significant price reductions, they might inadvertently reduce physical access while increasing insurance coverage for those same treatments. The retail price of a medication includes manufacturing, transportation, and pharmacy dispensing fees. Distribution in particular operates on very small profit margins, and price reductions have effects on the incentives for distribution to lower population density regions. The discussion noted that there are significant knowledge gaps among public servants regarding pharmaceutical distribution systems, benefit administration complexities, and market dynamics governing drug pricing and formulary management. Industry representatives noted that there are opportunities for better collaboration across the drug manufacturing, distribution, and dispensing supply chain and with governments.
The session concluded with broad recognition that effective pharmacare reform demands collaborative approaches that preserve successful existing arrangements while addressing genuine coverage gaps through targeted interventions rather than comprehensive system transformation. A practical approach must balance fiscal restraint and constraints with improving access to treatments, and incorporating complex market dynamics and unintended consequences into the consideration.
The Canadian Landscape – Different Approaches, Pros and Cons, and Comparative Advantages
The presentations for this session focused on existing provincial approaches to pharmacare in Quebec, Alberta, and Ontario. Each provincial system contains insights into efficiency, effectiveness, and potential approaches for future expansions of public drug insurance coverage.
The examination of Quebec’s mixed pharmacare model presented a balanced approach that achieves universal coverage while maintaining private insurance options. Since 1997, Quebec has operated a system ensuring all residents possess coverage either through the public plan administered by the Régie de l’assurance maladie du Québec or through mandatory private insurance when available through employers. This model preserves universality without eliminating private coverage, allowing individuals with employer-sponsored benefits to maintain their existing arrangements while providing public coverage for those without private access.
The Quebec system incorporates structured cost-sharing mechanisms through premiums, monthly deductibles, and coinsurance, distributing financial responsibility among individuals, employers, and government while maintaining long-term fiscal sustainability. Implementation data indicated that public spending remained consistent with Canadian averages following the program’s introduction, while overall per capita pharmaceutical expenditures increased through expanded private plan utilization rather than government spending growth. This cost distribution pattern was presented as evidence of enhanced sustainability compared to single-payer alternatives.
The presentation contrasted Quebec’s approach with federal pharmacare proposals estimated at over $40 billion annually when fully implemented, arguing that private plans typically cover two to three times more drugs and treatments than public programs. The analysis characterized public single-payer systems as inherently requiring service rationing through delays and restricted formularies to maintain fiscal predictability, presenting reduced coverage quality as a systemic feature rather than implementation oversight.
The next presentation gave provider and patient experience perspectives and emphasized pragmatic implementation challenges. OHIP+ implementation was framed as a cautionary example of hasty policy development.1010 OHIP+ was implemented near the end of a political cycle, and the presentation noted an upcoming election and the political pressure of an opposition platform as major contributors to the rapid implementation of OHIP+. The initial form of OHIP+ provided first-dollar, universal coverage for Ontario residents under the age of 25. At the time, policy commentary questioned the program’s efficiency, noting that most young people were already covered under their parents’ private insurance plans. The expansion of universal coverage mainly affected children and youth from low-income households and high school or post-secondary graduates who no longer qualified as “dependents” under their parents’ plans and also lacked independent employer-sponsored coverage (Busby and Blomqvist 2017).1111 Those who already had private coverage experienced no material changes in access. Some patients were disrupted if their medications were switched from their existing coverage to the public plan, similar to the disruptions experienced by patients during Manitoba’s implementation of contraceptive and diabetes coverage, discussed in the previous session. Following the provincial election, the program changed to a fill the gaps approach. The presentation highlighted administrative complexities during the initial rollout, including frontline provider confusion, patient coverage disruptions, and inadequate engagement with healthcare delivery stakeholders.
Since governments function as payors rather than direct drug purchasers, there are public service knowledge gaps regarding drug distribution systems. The presentation questioned the likelihood of further discounts from a universal program. The final retail price of a drug includes manufacturing and distribution costs, pharmacy overhead, and dispensing fees. The Pan-Canadian Pharmaceutical Alliance already negotiates on behalf of public insurance plans, and prices of patented medicines are regulated. While expanded public insurance plans could achieve some additional discounts, they would not be significant enough to offset growing costs. The presentation encouraged a collaborative approach with front-line providers and considered trade-offs between consumer affordability and accessibility.
Alberta’s experience provided insights into program evolution and political sensitivities surrounding benefit modifications. The presentation described Alberta’s legislative framework encompassing seniors’ coverage, universal access plans requiring enrollment and premium payments, and income-tested premium support programs. Implementation lessons revealed significant political resistance to benefit changes, particularly affecting seniors who constitute influential voting constituencies, resulting in minimal policy adjustments since 1994 despite changing economic circumstances.
The presentation cautioned that while it might be theoretically advisable to consolidate different provincial plans under a single program, the practical and political challenges of redefining benefits coverage are difficult to overcome. Alberta has various specialized programs addressing specific therapeutic areas, including cancer treatment coverage, HIV prevention programs, and insulin pump support, each providing comprehensive coverage for Alberta residents. Attempts to consolidate these programs encountered substantial stakeholder resistance, illustrating challenges inherent in modifying established benefit structures even when changes might improve administrative efficiency and not materially impact beneficiary coverage.
During the discussion, participants debated questions regarding provincial autonomy versus national standardization, exploring tensions between constitutional jurisdictional divisions and desires for consistent coverage standards. The discussion revealed skepticism about the federal government’s capacity to impose uniform approaches, given provincial resistance to centralized health administration and historical challenges in securing interprovincial cooperation on health policy initiatives.
The conversation explored the federal shift to bilateral agreements as an imperfect but potentially more effective mechanism for achieving coordination while preserving provincial decision-making authority, at least for the specific therapeutic categories included in the national pharmacare legislation. To be effective, however, bilateral agreements and provincial coverage must proliferate across all regions. First-dollar universal coverage is not compatible with Quebec’s existing mixed payor universal coverage model. In addition, the lack of federal commitments to continue making new bilateral agreements with provinces and 65 percent of the available funds already allocated suggests that national harmonization is unlikely under the current framework and budget. Participants discussed, with varying opinions, whether the existing pharmacare framework could be adapted to be more compatible with existing provincial programs and private insurance coverage, or whether the existing framework should be repealed and replaced, given the practical challenges and underfunding.
Given the real-life experience with OHIP+, some participants highlighted the importance of gradual, consultative approaches rather than broad, potentially disruptive system transformation. Conversely, the difficulty in integrating different programs in Alberta led some participants to urge for a longer-term strategic view to public program expansion. In particular, expansions to public insurance should build upon the foundation of existing programs to minimize new administrative costs and prevent long-term program fragmentation. In either case, the discussion highlighted communication challenges, noting that even well-designed programs require extensive public education to ensure effective utilization and stakeholder understanding.
Workshop Summary: Building Fiscally Responsible Pharmacare Systems
The workshop concluded with a discussion amongst participants about the future of pharmacare policy. The dual challenge of addressing population-wide access to pharmaceutical coverage and achieving fiscal sustainability of public programs serves as a practical anchor for future policy development. There were some areas of consensus among participants, but implementation details and the context of economic and political changes leave significant room for continuing to refine the approach to universal pharmacare access.
Areas of Consensus
The workshop revealed remarkable alignment across diverse stakeholders on several fundamental principles. Participants universally acknowledged the necessity of addressing coverage gaps for uninsured and underinsured Canadians. There was a strong consensus on preserving the existing dual public-private system, which serves approximately two-thirds of Canadians effectively, rather than pursuing a comprehensive system replacement.
Given the broader challenges in the healthcare system and limited fiscal capacity for healthcare investment, filling the gaps is the more practical approach. It has the benefit of minimizing disruption for people who already have insurance coverage and addresses access for the uninsured population. There was widespread agreement on the need for fiscal responsibility and sustainability in any policy reform, acknowledging that resource constraints necessitate strategic prioritization rather than expansive universal coverage approaches.
Participants also converged on the critical importance of maintaining Canada’s attractiveness as a destination for pharmaceutical investment and innovation. The discussion revealed shared concerns about regulatory efficiency and the need to preserve commercial confidence. Private insurance provides coverage for more medications and more rapid access to new medicines.
Critical Policy Issues
Several fundamental policy challenges emerged as central to the debate over pharmacare. The fiscal sustainability question proved particularly acute, with one participant noting that Ontario’s drug programs grow at 8 percent annually, with some components expanding at annual rates of 20 percent. This cost escalation raises serious questions about long-term affordability and the appropriate balance between public and private financing mechanisms.1212 The largest cost driver for public and private drug plans is increasing use of higher-cost drugs (drug-mix effect), followed by demographic change (population growth and aging) (Zhang 2024).
The workshop identified significant tensions between access to innovative medicine and cost containment. Participants emphasized that Canada’s “unacceptable time to market” for new therapies undermines the country’s position as an attractive pharmaceutical market, potentially limiting patient access to cutting-edge treatments. This challenge is compounded by concerns that single-payer models may lead to rationing and diminished access through restrictive formularies. A growing share of prescription drug expenditures is being dedicated to high-cost treatments for relatively few beneficiaries, for both public and private insurance programs. Participants noted the need for a more comprehensive strategy to address access and affordability trade-offs associated with rare and high-cost treatments.
The role and scope of federal institutions, particularly the Canadian Drug Agency (CDA), emerged as a contentious issue. Participants questioned whether the CDA should expand beyond health technology assessment into broader regulatory functions, with concerns that institutional mission creep would undermine core competencies.
Provincial-federal relations represent another critical dimension, with participants noting that provincial governments remain reluctant to cede authority to federal oversight. The existing bilateral agreement approach has created implementation inconsistencies across jurisdictions, raising questions about national coordination while respecting provincial jurisdiction.
Areas of Debate
The most significant debate centred on the best approach for achieving universal pharmacare coverage in the current legislative context and future policy uncertainty. The legislation sets a foundation of coverage by indication and a precedent for first-dollar universal coverage. Some participants questioned whether the best approach would be to repeal the existing legislation and replace it with funding to address coverage gaps. The current policy appears to be stalled with no federal commitment to new bilateral agreements. The existing funding commitments are time-limited, adding additional uncertainty about the longer-term fiscal liability for provinces that are receiving funding for diabetes and contraception coverage. Meanwhile, the CDA reports that a national bulk purchasing strategy and formulary are being finalized.
Participants debated whether current policy momentum can be redirected or whether stakeholders should focus on implementation improvements within existing frameworks. Institutional design questions proved contentious, particularly regarding the CDA’s evolving mandate. While participants agreed on the need for streamlined processes, they disagreed on whether expanded federal coordination would improve efficiency or create additional bureaucratic complexity.
A particularly nuanced discussion emerged regarding public communication challenges. Participants acknowledged that advocates for universal single-payer systems benefit from simpler messaging that promises comprehensive coverage without acknowledging trade-offs or implementation complexities. They also recognized that explaining nuanced policy positions that balance multiple objectives poses a significant communication challenge in the broader public debate on pharmacare. The appropriate level of user cost-sharing generated substantial discussion, with participants questioning optimal copayment structures and the role of private insurance in managing high-cost drug volatility.
Conclusion
The workshop demonstrated that while stakeholders share fundamental objectives regarding coverage expansion and system sustainability, significant strategic and implementation debates represent opportunities for further study and discussion. The consensus around targeted gap-filling approaches and system preservation provides a foundation for policy development, but questions regarding advocacy strategy, institutional design, and federal-provincial coordination require continued engagement. The emphasis on avoiding patient disruption while maintaining access to innovation suggests that incremental, evidence-based reforms may prove more viable than comprehensive system transformation, although the political economy of such approaches remains complex and contested.
Given limited government funds for new programs and many competing health policy and delivery priorities, there was consensus that federal pharmacare funding should focus on reducing coverage gaps across public plans and reducing out-of-pocket costs for the minority of Canadians who are un- or under-insured.
The session concluded with the recognition that successful pharmacare reform requires acknowledging existing system strengths, paying careful attention to implementation complexities, and respecting provincial jurisdictional authority while addressing genuine coverage gaps through targeted, evidence-based interventions rather than ideologically driven system transformation.
References
Barkova, Lisa. 2024. “An Act Respecting Pharmacare.” Parliamentary Budget Office. https://www.pbo-dpb.ca/en/publications/LEG-2425-003-S--an-act-respecting-pharmacare--loi-concernant-assurance-medicaments.
Barkova, Lisa, and Carleigh Busby. 2023. “Cost Estimate of a Single Payer Universal Drug Plan.” Parliamentary Budget Office. https://www.pbo-dpb.ca/en/publications/RP-2324-016-S--cost-estimate-single-payer-universal-drug-plan--estimation-couts-un-regime-assurance-medicaments-universel-payeur-unique.
Busby, Colin, and Ake Blomqvist. 2017. “Here’s a Better Plan for OHIP+.” Intelligence Memo. Toronto: C.D. Howe Institute. https://cdhowe.org/publication/busby-and-blomqvist-heres-better-plan-ohip/.
Coletto, David. 2023. “Canadians and Health Care: Workplace and Group Insurance Plans.” Abacus Data. https://abacusdata.ca/healthcare-canadians-clhia-workplace-and-group-insurance-plans/.
Department of Finance Canada. 2024. Budget 2024: Fairness for Every Generation. Chapter 2. Ottawa: Government of Canada. https://budget.canada.ca/2024/report-rapport/chap2-en.html.
Froese, Ian. 2025. “3 Provinces, 1 Territory Made Pharmacare Deals. Ottawa Won’t Say if Others Are Coming.” CBC News. https://www.cbc.ca/news/politics/pharmacare-four-provinces-territories-implementation-deals-1.7614040.
Health Canada. 2023. “Drugs for Rare Diseases Bilateral Agreements.” March 22. https://www.canada.ca/en/health-canada/corporate/transparency/health-agreements/shared-health-priorities/drugs-for-rare-diseases-bilateral-agreements.html.
Health Canada. 2024. “National Pharmacare Bilateral Agreements.” October 10. https://www.canada.ca/en/health-canada/corporate/transparency/health-agreements/national-pharmacare-bilateral-agreements.html.
Robson, William, and Rosalie Wyonch. 2019. Filling the Gaps: A Prescription for Universal Pharmacare. Commentary 544. Toronto: C.D. Howe Institute. https://cdhowe.org/publication/filling-gaps-prescription-universal-pharmacare/.
Wyonch, Rosalie. 2025. “Trump’s Pharma Pricing Order Could Have Big Canadian Consequences.” Intelligence Memo. Toronto: C.D. Howe Institute. https://cdhowe.org/publication/trumps-pharma-pricing-order-could-have-big-canadian-consequences/.
Yang, Fei-Ju, and Shikha Gupta. 2024. Exploring Gaps in Prescription Drug Insurance Coverage Among Men and Women in Canada Using an Intersectional Lens. Statistics Canada. https://www150.statcan.gc.ca/n1/pub/75-006-x/2024001/article/00001-eng.pdf.
Zhang, Yvonne. 2024. Cost Drivers for Canada’s Public and Private Drug Plans During the COVID-19 pandemic: A 2019-2022 Comparative Analysis. Canadian Association for Health Services and Policy Research. https://www.canada.ca/en/patented-medicine-prices-review/services/npduis/analytical-studies/posters/cahspr2024-cost-drivers.html.
Attendee List
Convener:
• Rosalie Wyonch, C.D. Howe Institute
Members:
• Kerry Allerton
• Ashlee Babcock
• Justin Bates
• Jeff Beach
• Angela Behboodi
• Patricia Caetano
• Patrick Dicerni
• Michael Dietrich
• Alex Dobrescu
• Jacqueline Dobson
• Daniel Dufort
• Joe Farago
• Laura Fitzgerald
• Neil Fraser
• Alaine Grand
• Bettina Hamelin
• Erika Hatherly
• Madeline Hieneman
• Fred Horne
• Lincoln Kim
• Vince Lamanna
• Ashley McVey
• Chad Mitchell
• Charlini Nicholapillai
• Erin Polka
• Christopher Praught
• David Veillette
• Gary Walters
• Joan Weir
• Simona Zar
• Tingting Zhang
Related Publications
- Research

